
Looking for a clinical trial that's right for you?
Find a trialUCI Health, your source for clinical trials
UCI Health has one of Orange County’s largest clinical trials programs, with 1,000-plus trials and 3,450 patients enrolled in the past year. By participating in a clinical trial, you help shape the future of medicine. Help us discover and cure!
What are clinical trials?
Clinical trials evaluate the effects of new interventions on health.
Why participate?
Discovery depends on you!

Get involved!
Become a partner in discovering new clinical therapies.
Featured Trials

A Phase 2b Study Evaluating Safety of Oral TAK-279 in Moderate to Severe Crohn's Disease
The drug is being tested to treat participants with moderately to severely active Crohn's disease. The study will look at the efficacy and safety of the drug and will enroll approximately 268 participants.

A Phase 3, Randomized, Double-Blind, Placebo-Controlled Program to Evaluate MK-7240
A Study to Evaluate the Efficacy and Safety of MK-7240 in Participants with Moderately to Severely Active Ulcerative Colitis.
A study to evaluate the efficacy and safety of JCELL in patients with retinitis
The primary objective of this Phase 2/3 study is to evaluate the efficacy, safety and tolerability of a single intravitreal injection of jCell (famzeretcel) for the Treatment of Retinitis Pigmentosa (RP).

A Phase 1 Study of FT819 in Participants with Moderate to Severe Active Systemic Lupus Erythematosus.
This is a Phase 1 study designed to evaluate the safety, pharmacokinetics (PK), and anti-B-cell activity of FT819 following treatment with or without auxiliary medicinal product (AMP) in participants with moderate to severe active systemic lupus erythematosus (SLE). The study will consist of a dose-escalation stage, followed by an expansion stage to further evaluate the safety and activity of FT819.

Lung Cancer Screening of Family Members of Patients with Mutation-Driven Lung Cancer
The purpose of this research study is to find out whether researchers can help find lung cancer early by screening immediate family members of patients with lung cancer who have a genetic mutation by undergoing a low dose computed tomography (CT) scan.

Repurposing Riluzole for Cancer-Related Cognitive Impairment: a Pilot Trial
A phase 2a, randomized, double-blinded, placebo-controlled pilot clinical trial determining the impact of Riluzole therapy on circulating brain derived neuropathic factor (BDNF) levels of breast cancer survivors with cancer related cognitive impairment.
Advancing Spine Care
Advancing Spine Care
Learn more about clinical trials at UCI Health
Explore valuable resources and support for all aspects of clinical trials at UCI health

UCI Research News
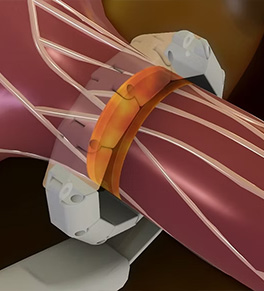
First U.S. test of nerve-blocking device for resistant hypertension
UCI Health urologist Dr. Pengbo Jiang recently performed the first U.S. procedure using radiofrequency electrodes to block nerves surrounding the renal arteries without damaging the blood vessel’s integrity.

UC Irvine Anti-Cancer Challenge
The UC Irvine Anti-Cancer Challenge is a community movement to raise awareness and funds for critical cancer research at the UCI Health Chao Family Comprehensive Cancer Center and CHOC, part of Rady Children’s Health.
Every participant-raised dollar directly funds pilot studies and early phase clinical trials that aim to develop new insights into cancer prevention, treatment and cures — to save lives.
Every participant-raised dollar directly funds pilot studies and early phase clinical trials that aim to develop new insights into cancer prevention, treatment and cures — to save lives.

Advancing regenerative medicine therapies
University and community guests recently gathered to celebrate the 7,700 square-foot Good Manufacturing Practice facility’s grand opening in Hewitt Hall’s basement on the UC Irvine campus.




