Finally, a possible long-term treatment for type 1 diabetes

For decades, scientists have searched for a way to transplant healthy pancreatic islet cells into the bodies of people with type 1 diabetes.
The disease occurs when the body’s immune system attacks and destroys islet cells, rendering them unable to make insulin and other hormones that regulate blood sugar levels.
Now, after many setbacks and disappointments, UCI Health researchers are studying a new approach in a human clinical trial — one of only six sites in the world to evaluate the treatment.
The phase 1/2 study will determine whether the islet cell replacement therapy, a device called PEC-Direct, is safe and can provide blood glucose control in a manner similar to an artificial pancreas.
Living without injections
The goal is to develop a therapy for type 1 diabetes that does not depend on long-term use of insulin in the form of injections or insulin pumps. Some 1.25 million Americans are living with type 1 diabetes, including about 200,000 youth (less than 20 years old) and more than 1 million adults (20 years and older).
“Right now, unfortunately, there is no cure,” says endocrinologist Dr. Ping H. Wang, medical director of the UCI Health Diabetes Center and lead investigator for the UCI Health arm of the PEC-Direct trial.
“That means the patient has to use insulin to control blood sugar. Insulin is a very sensitive hormone. The dose has to be exactly right. These patients are constantly under stress due to changes in blood sugar.”
Wang is conducting the first-in-human study to evaluate the safety of the islet-cell delivery system for patients with type 1 diabetes at the UCI Alpha Clinic, the clinical trials arm of the UCI Sue & Bill Gross Stem Research Center.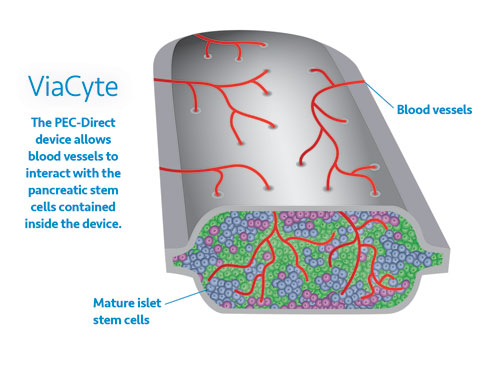
A permanent, stable insulin source
The PEC-Direct treatment aims to provide a permanent, stable source of insulin. The islet cells in the device — called pancreatic beta precursor cells — are derived in the lab from human embryonic stem cells and placed in a capsule that is implanted under the skin in the abdomen (a few stitches are required).
The device releases insulin and other hormones into the patient’s bloodstream.
“In the last year or two, there has been renewed interest in islet cell transplantation because of investments in technology in how cells are prepared,” he says.
“We have reached a new stage where we can obtain islet cells from human embryonic stem cells, which is a big step. This unlimited supply of islet cells is needed to make this type of treatment a reality.”
Immune suppression to prevent rejection
The study will include adult patients with type 1 diabetes who have struggled with control and are at high risk for complications from the disease.
Study participants will keep track of their blood glucose and insulin usage. They will take immunosuppressant medications to keep the body from rejecting the device, which will be implanted by UCI Health transplant surgeon Dr. Hirohito Ichii.
“Immune suppression is definitely one of the major hurdles we have to overcome,” Wang says.
“The cell is an embryonic stem cell, and therefore it’s not the patient’s own cell. But the preliminary studies so far show this capsule seems to survive better than a pure islet cell transplant itself.”
A path to 'an ultimate cure'
In the future, however, a device that does not trigger rejection may become possible, Wang says.
The maker of PEC-Direct, ViaCyte Inc., is developing genetically engineered stem cells that will not trigger an immune response.
The cells are being engineered to edit out the cell surface proteins that interact with the immune system.
“In doing this trial, we are creating the path forward so eventually when immune-evasive stem cells become available, that will potentially provide an ultimate cure,” Wang says.
The UCI Alpha Clinic is the clinical trials arm of the UCI Sue & Bill Gross Stem Cell Research Center (SCRC) and part of a network of the state's leading medical centers funded by the California Institute of Regenerative Medicine (CIRM). The ASCC specializes in delivering leading-edge stem cell clinical trials to patients and seeks to accelerate the development of treatments through partnerships with patients, medical providers and clinical trial sponsors. The ASCC network supports both CIRM-funded clinical trials and those funded by academic and industry sponsors. Visit www.stemcell.uci.edu to learn more about ASCC, clinical stem cell trials and regenerative medicine research at UCI.
Explore further
Browse more blog posts by topic.




