
Looking for a clinical trial that's right for you?
Find a trialUCI Health, your source for clinical trials
UCI Health has one of Orange County’s largest clinical trials programs, with 1,000-plus trials and 3,450 patients enrolled in the past year. By participating in a clinical trial, you help shape the future of medicine. Help us discover and cure!
UCI Clinical Trials
Partnered with our leading academic medical center, we offer top-tier care and access to leading-edge trials from across the nation. We are dedicated to serving patients, researchers and sponsors with unwavering commitment and excellence.
What are clinical trials?
Why participate?

Get involved!
Featured Trials

Phase 3 Study to Evaluate Remibrutinib in Patients with Generalized Myasthenia Gravis

A Phase 3 Study of Telitacicept in Patients with Generalized Myasthenia Gravis
A study to evaluate the efficacy and safety of JCELL in patients with retinitis

A Phase 1 Study of FT819 in Participants with Moderate to Severe Active Systemic Lupus Erythematosus.

Lung Cancer Screening of Family Members of Patients with Mutation-Driven Lung Cancer

Repurposing Riluzole for Cancer-Related Cognitive Impairment: a Pilot Trial
GLP-1 Drugs
In this episode, UCI Health experts Dr. Qin (Kin) Yang, Dr. Andy Lee, and Professor Nathan Wong discuss GLP-1 medications like Ozempic, Wegovy, Mounjaro, and Zepbound—exploring their benefits for weight loss, diabetes, and heart disease, as well as the broader cost and access implications.
Live Well with UCI Health on iHeart Radio: GLP-1 discussion with Dr. Qin Yang, Dr. Andy Lee and Professor Nathan Wong
Advancing Spine Care
Advancing Spine Care
Learn more about clinical trials at UCI Health
Explore valuable resources and support for all aspects of clinical trials at UCI health

UCI Research News
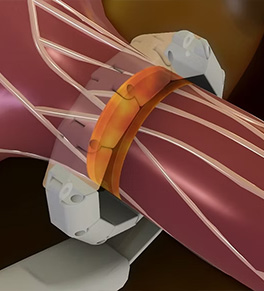
First U.S. test of nerve-blocking device for resistant hypertension

Lung cancer screening is essential for early detection, treatment





