Dr. Claire Henchcliffe is encouraged by promising clinical trial results for a stem-cell infusion therapy that could stop the progression of Parkinson’s disease.
Photo credit: Michael Neveux.
After decades of researching and treating Parkinson's disease, UCI Health neurologist Dr. Claire Henchcliffe reports promising results a first-in-human clinical trial of stem cells engineered to replace dopamine-producing neurons destroyed by the disease.
The hope is that infusing the brain with these specialized neural cells — called bemdaneprocel (BRT-DA01) and developed by BlueRock Therapeutics — will restore critical dopamine production and alleviate the progression of Parkinson’s disease, says Henchcliffe, a principal investigator of the novel study along with colleagues at Weill Cornell Medicine in New York City and the University of Toronto.
The study was conducted under the auspices of the UCI Alpha Clinic, the clinical trial arm of the UCI Sue and Bill Gross Stem Cell Research Center.
An international Parkinson’s disease expert and chair of the UCI School of Medicine’s Department of Neurology, Henchcliffe answers questions about the study and its findings.
Q.: What was the study designed to do?
This phase 1 clinical trial tested whether an injection of bemdaneprocel stem cells into the brains of 12 patients with advanced Parkinson’s disease was safe and well tolerated. Patients also received immunosuppression treatment to prevent rejection.
Q: What makes this study unique?
Huge advances in technology make it possible to turn precursor cells, derived from pluripotent stem cells, into dopamine-producing neurons. We now can grow an almost unlimited number of these cells with a high degree of quality control.
Q: What is Parkinson’s disease?
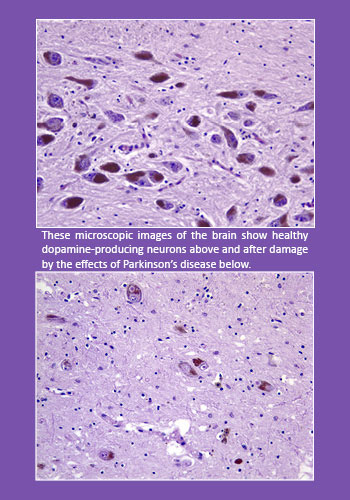 Parkinson’s disease is a progressive neurodegenerative disorder affecting neurons in the part of the brain called the substantia nigra. These neurons produce dopamine, a neurotransmitter that allows the brain and nervous system to control and coordinate body movements.
Parkinson’s disease is a progressive neurodegenerative disorder affecting neurons in the part of the brain called the substantia nigra. These neurons produce dopamine, a neurotransmitter that allows the brain and nervous system to control and coordinate body movements.
Many Parkinson’s symptoms — tremors, muscle stiffness, slow movement, impaired balance and coordination — occur when these dopamine-producing neurons become impaired or die.
The disorder — which affects an estimated 1 million Americans, more men than women, and mostly occurs in people over age 60 — may also cause a range of other symptoms, including difficulty swallowing, chewing and speaking, cognitive decline, urinary problems or constipation, anxiety and depression. While the progressive disease, isn’t fatal itself, it does increase the risk of falls, pneumonia and other conditions that can result in death.
Q: What did the clinical trial results show?
The treatment proved to be safe for patients with advanced Parkinson’s and did not cause significant side effects. We’re also a little bit excited because specialized imaging scans showed that the injected cells not only survived but also appear to have become integrated with the patients’ neural cells. Although the trial was not designed to test the therapy’s efficacy, 11 of the 12 patients showed some improvement in motor symptoms.
If the treatment is successful in subsequent trials, it could be used earlier and earlier. We would love to see it developed for early-stage disease, which would mean exposing fewer patients to current Parkinson’s medications and their side effects.
Why get the therapy if medications control the disease?
Drugs used to replace dopamine or dopamine-like substitutes work well in early Parkinson’s, but their beneficial effects don’t last. People eventually need higher, more frequent doses. That can lead to uncontrolled, involuntary movements called dyskinesia.
This cell therapy has the potential to be a single, one-size-fits-all treatment, providing lifelong relief from Parkinson’s motor symptoms. We could then focus on other disease symptoms, such as depression and anxiety, pain, cognitive decline and sleep problems.
What is the next step?
We will continue to follow our phase 1 patients for another two years. BlueRock is designing a phase 2 clinical trial to test the treatment in a greater number of patients. We hope the Alpha Clinic and the Sue and Bill Gross Stem Cell Research Center will be part of that trial, which is expected to begin later this year.
Is UCI Health studying other leading-edge Parkinson’s therapies?
We have many clinical trials underway or coming soon for Parkinson’s disease, including a novel treatment my colleague Dr. Nicolás Phielipp will be conducting to infuse patients with their own neural cells, which have been modified to produce dopamine. This could avoid the immunosuppression therapy needed with bemdaneprocel.
The combination of the excellent care our Parkinson's Disease and Movement Disorders Program provides our patients, along with our experimental approaches, is something few other medical institutions can offer in Orange County or elsewhere.
About the UCI Alpha Clinic: The clinical trial arm of the UCI Sue & Bill Gross Stem Cell Research Center, the UCI Alpha Clinic is part of a network of academic medical centers funded by the California Institute for Regenerative Medicine (CIRM). The Alpha Clinic offers leading-edge stem cell clinical trials and gene therapy to patients. it also seeks to accelerate the development of new treatments through partnerships with patients, medical providers and clinical trial sponsors. Visit stemcell.uci.edu to learn more about our regenerative medicine research.
Related stories



